Two Festuca Species—F. arundinacea and F. glaucescens—Differ in the Molecular Response to Drought, While Their Physiological Response Is Similar
Abstract
:1. Introduction
2. Results
2.1. Genomic Discrimination and Species Identity of F. arundinacea and F. glaucescens
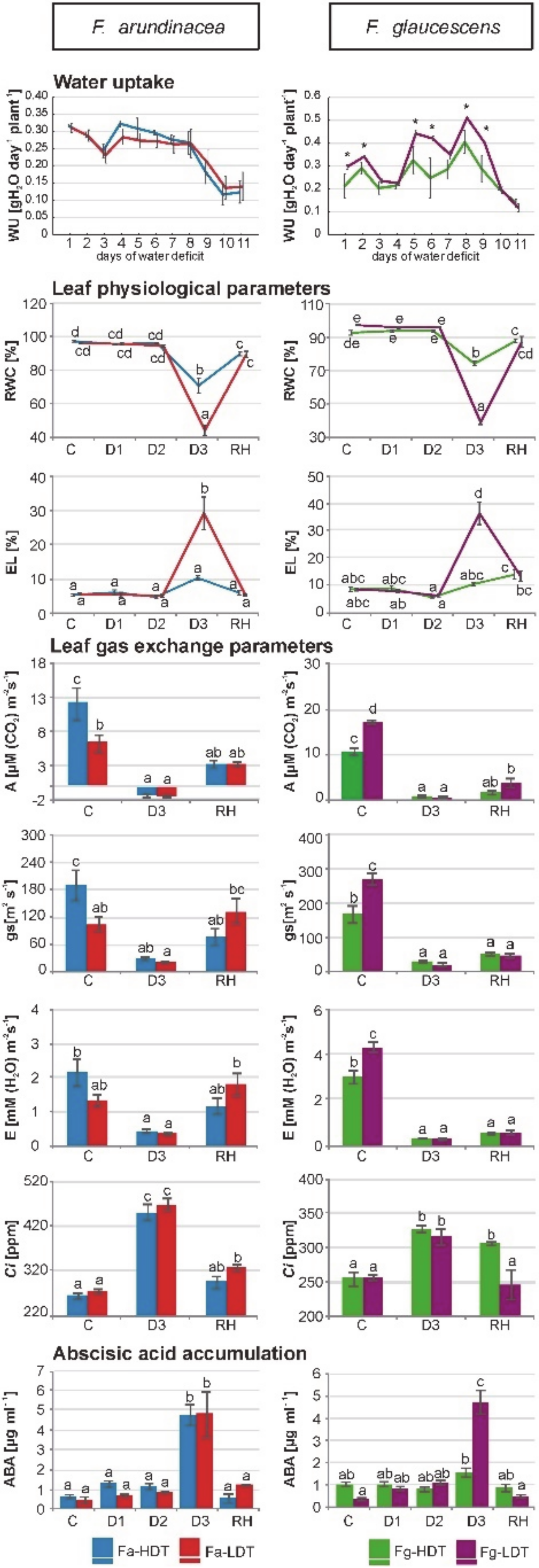
2.2. Physiological Parameters
2.3. ABA Content
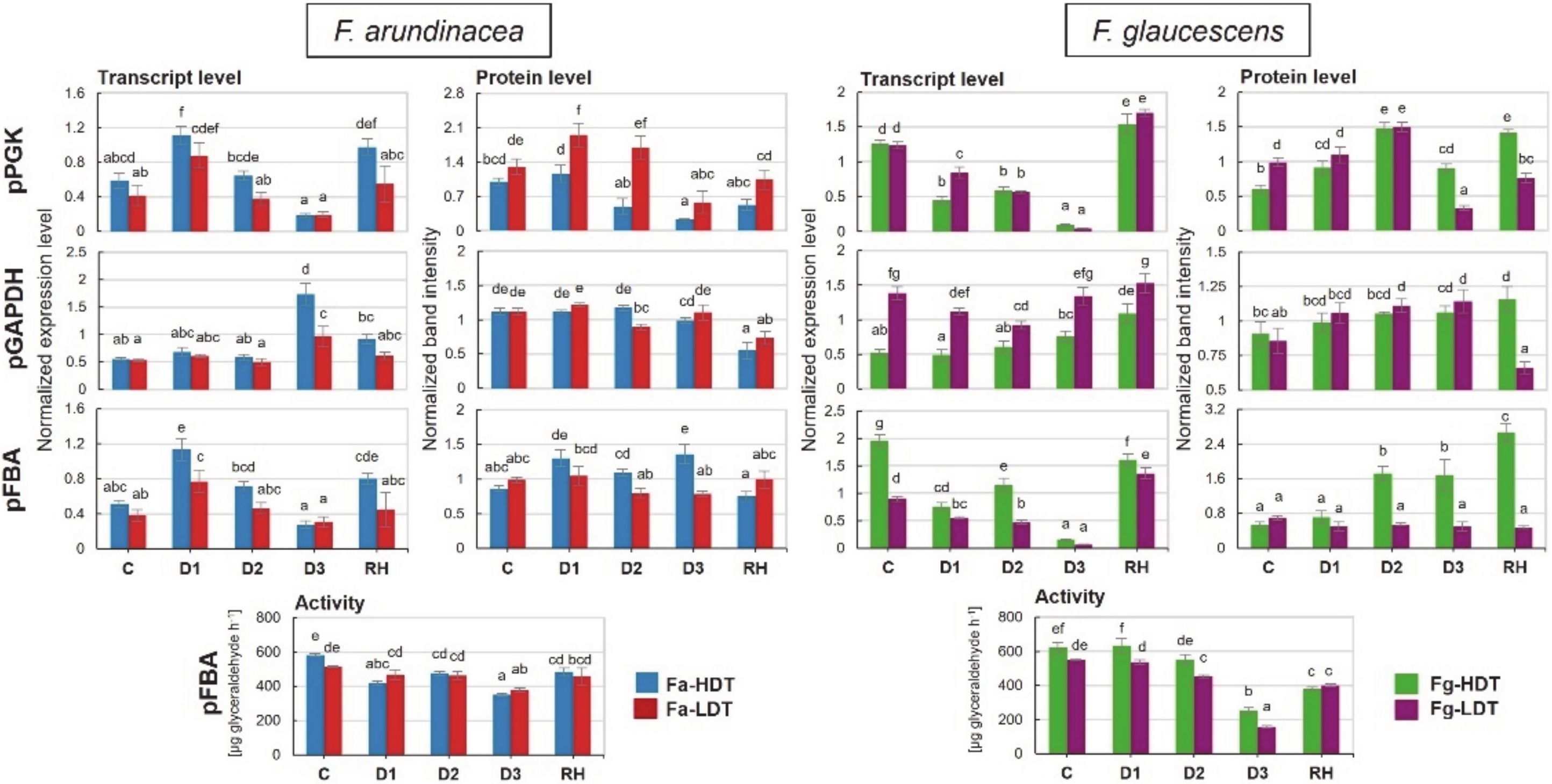
2.4. Expression of Genes Encoding the Calvin Cycle Enzymes at Transcript and Protein Levels
2.5. Activity of Chloroplast Aldolase
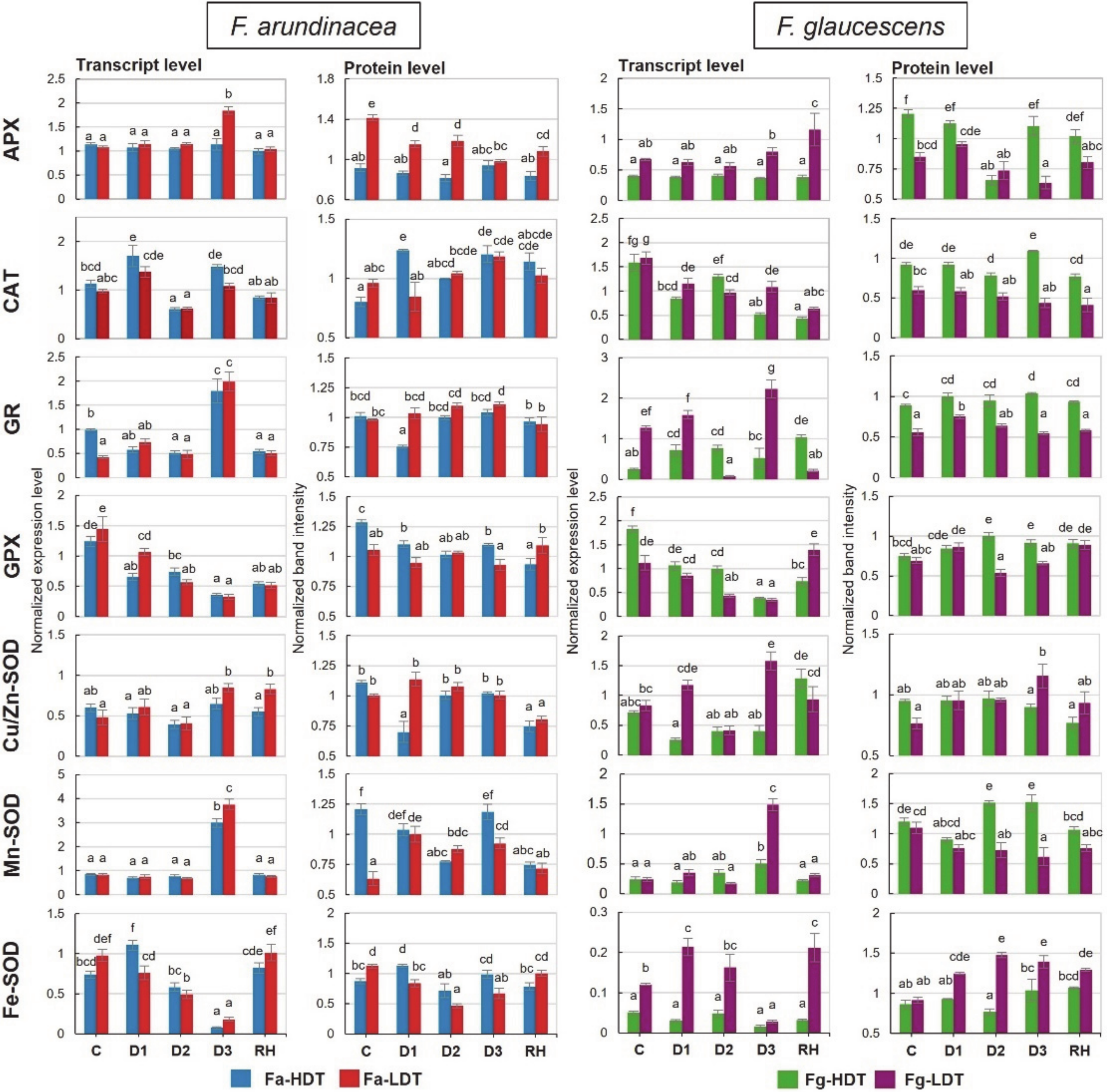
2.6. Expression of Genes Encoding Antioxidant Enzymes at Transcript and Protein Levels
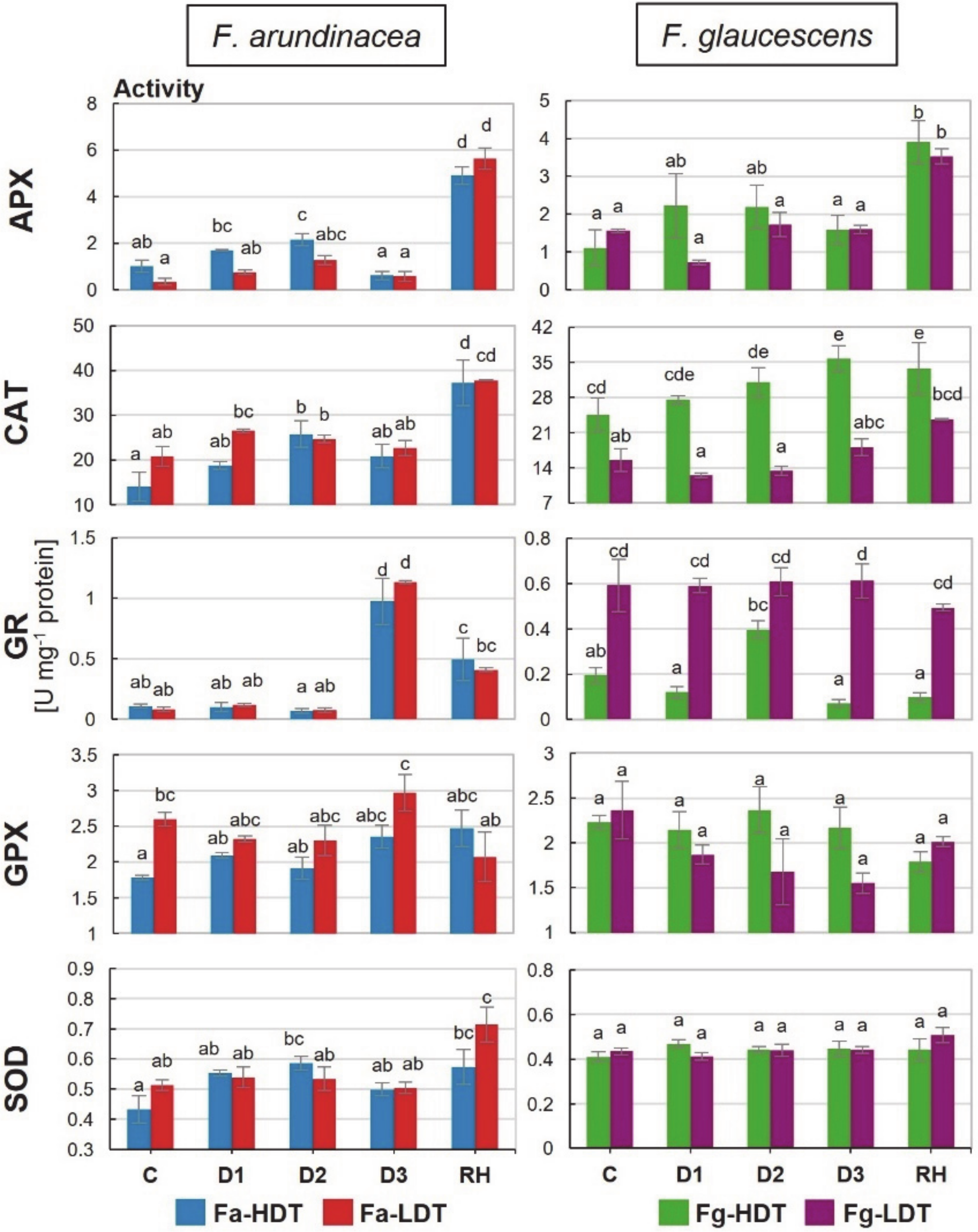
2.7. Activities of Antioxidant Enzymes
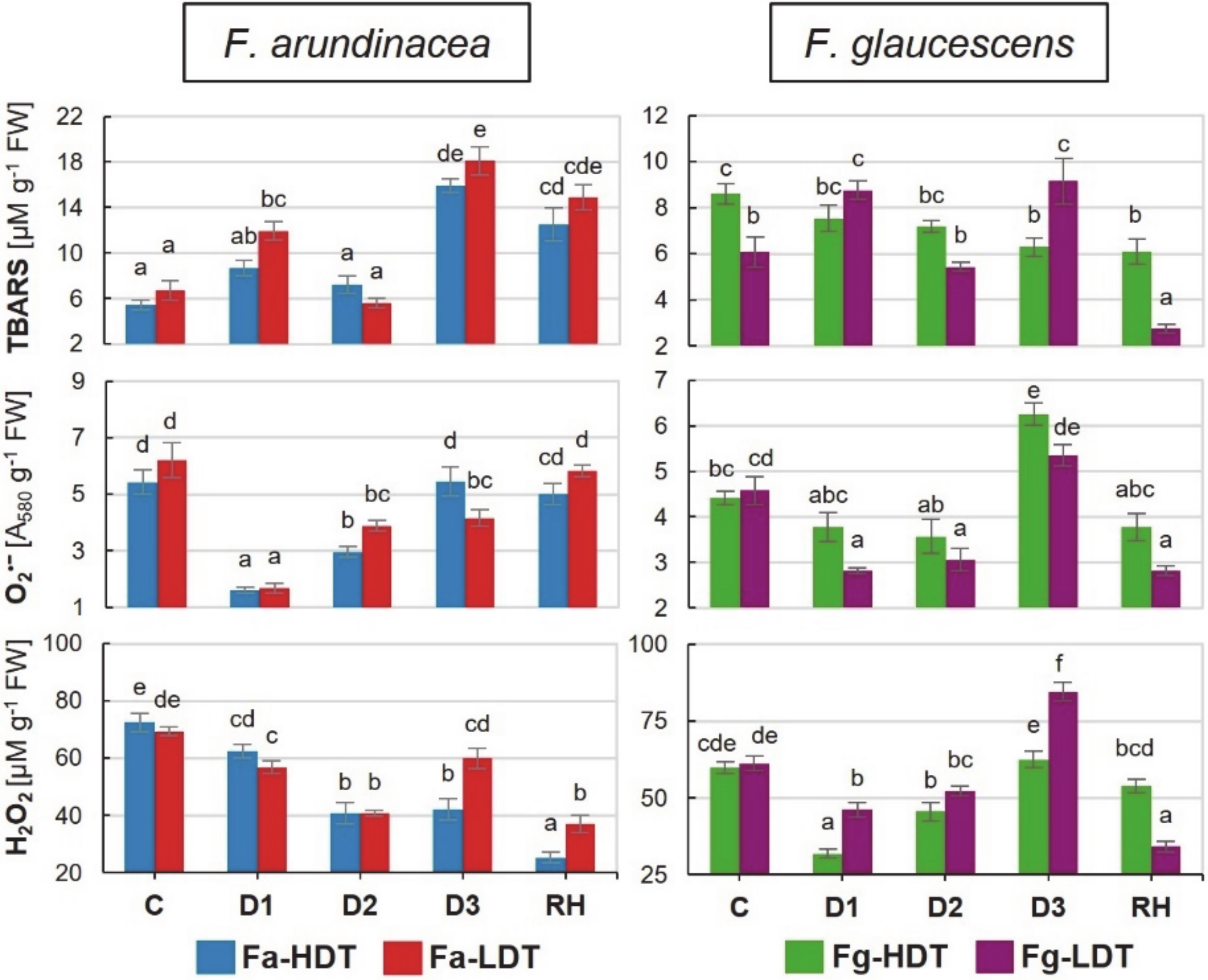
2.8. Lipid Peroxidation
2.9. Superoxide Anion Radical and Hydrogen Peroxide Contents
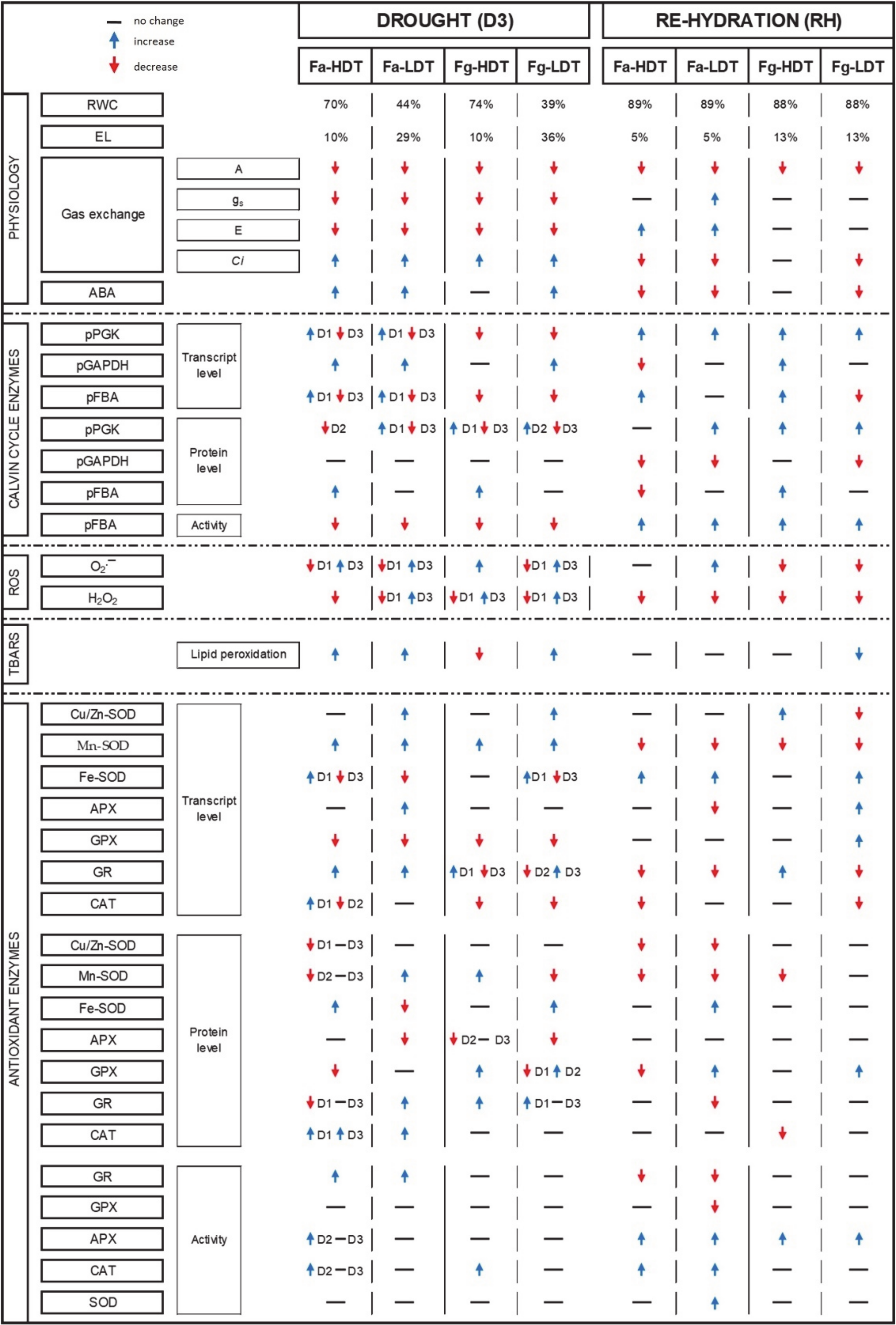
3. Discussion
3.1. Physiological Response to Drought
3.2. ROS Production and Performance of Enzymatic Antioxidant System
3.3. Efficiency of the Calvin Cycle
4. Conclusions
5. Methods
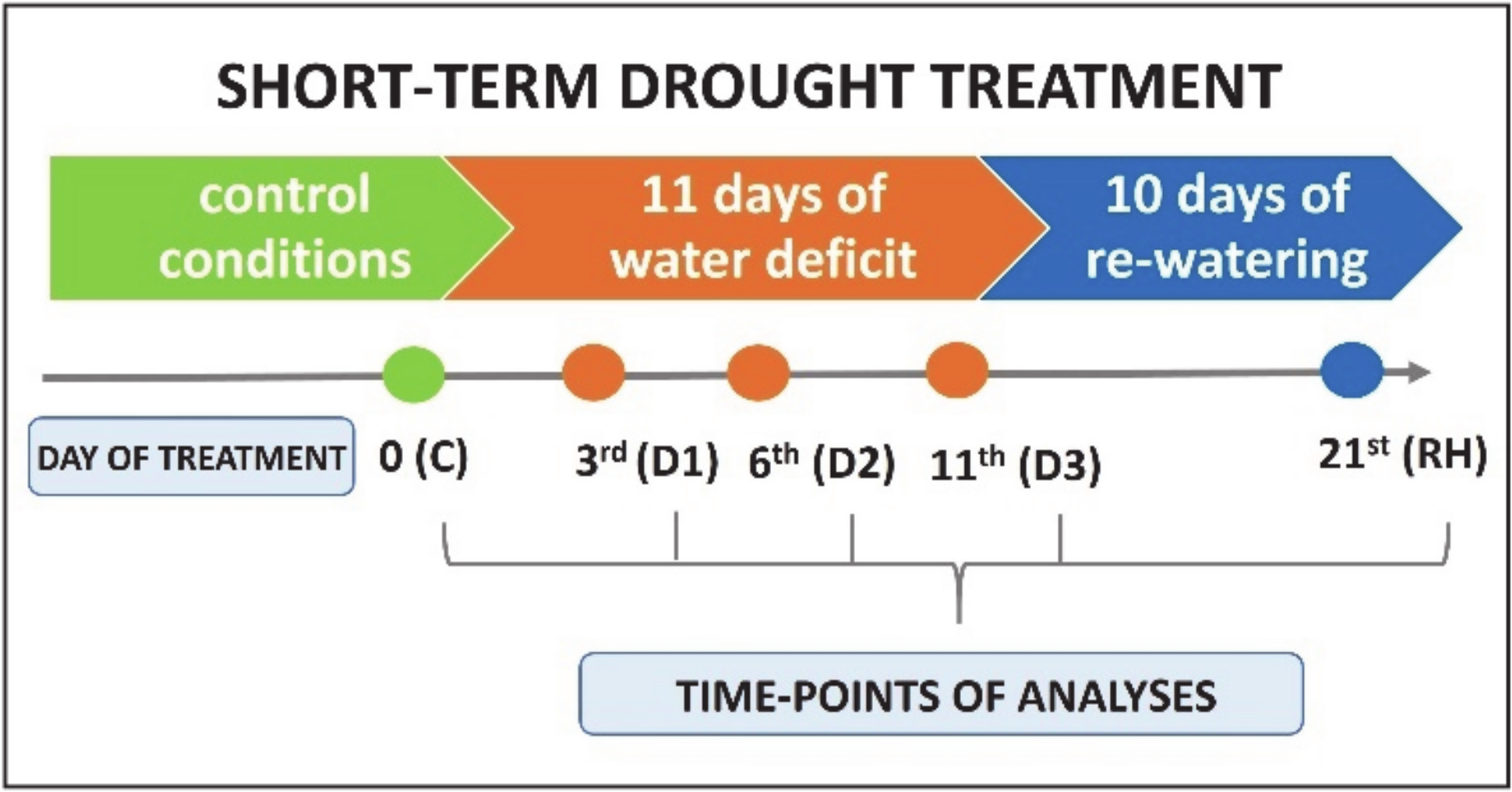
5.1. Plant Material, Growth Conditions, and Experimental Design
5.2. Fluorescent in Situ Hybridization (FISH)
5.3. Physiological Parameters
5.4. Accumulation of Abscisic Acid
5.5. Transcript Accumulation of the Calvin Cycle and Antioxidant Enzymes
5.6. Protein Accumulation of the Calvin Cycle and Antioxidant Enzymes
5.7. Activity of Chloroplast Aldolase
5.8. Activity of Antioxidant Enzymes
5.9. Lipid Peroxidation (TBARS Assay)
5.10. Production of Superoxide Anion Radical and Hydrogen Peroxide
5.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A | CO2 assimilation |
| ABA | abscisic acid |
| APX | L-ascorbate peroxidase |
| BSA | bovine serum albumin |
| CAT | catalase |
| Ci | CO2 concentration inside the leaves (interial) |
| Cu/Zn-SOD | chloroplastic Cu/Zn superoxide dismutase |
| DTT | DL-dithiothreitol |
| DW | leaf dry weight |
| E | transpiration |
| EDTA | ethylenediaminetetraacetic acid |
| EL | electrolyte leakage |
| Fe-SOD | Fe-dependent superoxide dismutase |
| FISH | Fluorescent in situ hybridization |
| FW | leaf fresh weight |
| gs | stomatal conductance |
| GPX | glutathione peroxidase |
| GR | glutathione reductase |
| GSH | reduced glutathione |
| HDT | high drought tolerant |
| L1 | electrical conductivity of the fresh leaf exudate |
| L2 | electrical conductivity of the leaf exudate immersed in liquid nitrogen |
| LDT | low drought tolerant |
| Mn-SOD | manganese superoxide dismutase |
| SDS | sodium dodecyl sulfate |
| SOD | superoxide dismutase |
| pFBA | plastid fructose-1,6-bisphosphate aldolase |
| pGAPDH | plastid glyceraldehyde-3-phosphate dehydrogenase |
| pPGK | plastid phosphoglycerate kinase |
| PPFD | photosynthetic photon flux density |
| PRL | 4-(2′-pyridylazo)-resorcinol |
| PSII | photosystem II |
| PTO | potassium sodium tartrate tetrahydrate |
| ROS | reactive oxygen species |
| RT | room temperature |
| RWC | relative water content |
| TBARS | thiobarbituric-reactive substances |
| WU | water uptake |
References
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, A.H.; Collins, R.P.; Humphreys, M.W.; Scullion, J. A new emphasis on root traits for perennial grass and legume varieties with environmental and ecological benefits. Food Energy Secur. 2016, 5, 26–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustyniak, A.; Perlikowski, D.; Rapacz, M.; Kościelniak, J.; Kosmala, A. Insight into cellular proteome of Lolium multiflorum/Festuca arundinacea introgression forms to decipher crucial mechanisms of cold acclimation in forage grasses. Plant Sci. 2018, 272, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, A.; Bocian, A.; Rapacz, M.; Jurczyk, B.; Zwierzykowski, Z. Identification of leaf proteins differentially accumulated during cold acclimation between Festuca pratensis plants with distinct levels of frost tolerance. J. Exp. Bot. 2009, 60, 3595–3609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosmala, A.; Perlikowski, D.; Pawłowicz, I.; Rapacz, M. Changes in the chloroplast proteome following water deficit and subsequent watering in a high and a low drought tolerant genotype of Festuca arundinacea. J. Exp. Bot. 2012, 63, 6161–6172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawłowicz, I.; Rapacz, M.; Perlikowski, D.; Gondek, K.; Kosmala, A. Abiotic stresses influence the transcript abundance of PIP and TIP aquaporins in Festuca species. J. Appl. Genet. 2017, 58, 421–435. [Google Scholar] [CrossRef] [Green Version]
- Pawłowicz, I.; Waśkiewicz, A.; Perlikowski, D.; Rapacz, M.; Ratajczak, D.; Kosmala, A. Remodeling of chloroplast proteome under salinity affects salt tolerance of Festuca arundinacea. Photosynth. Res. 2018, 137, 475–492. [Google Scholar] [CrossRef]
- Perlikowski, D.; Czyżniejewski, M.; Marczak, Ł.; Augustyniak, A.; Kosmala, A. Water deficit affects primary metabolism differently in two Lolium multiflorum/Festuca arundinacea introgression forms with a distinct capacity for photosynthesis and membrane regeneration. Front. Plant Sci. 2016, 7, 1063. [Google Scholar] [CrossRef] [Green Version]
- Perlikowski, D.; Augustyniak, A.; Masajada, K.; Skirycz, A.; Soja, A.M.; Michaelis, Ä.; Wolter, G.; Kosmala, A. Structural and metabolic alterations in root systems under limited water conditions in forage grasses of Lolium-Festuca complex. Plant Sci. 2019, 283, 211–223. [Google Scholar] [CrossRef]
- Perlikowski, D.; Augustyniak, A.; Skirycz, A.; Pawłowicz, I.; Masajada, K.; Michaelis, Ä.; Kosmala, A. Efficient root metabolism improves drought resistance of Festuca arundinacea. Plant Cell Physiol. 2020, 61, 492–504. [Google Scholar] [CrossRef]
- Humphreys, M.W.; Thomas, H.M.; Morgan, W.G.; Meredith, M.R.; Harper, J.A.; Thomas, H.; Zwierzykowski, Z.; Ghesquière, M. Discriminating the ancestral progenitors of hexaploid Festuca arundinacea using genomic in situ hybridization. Heredity 1995, 75, 171–174. [Google Scholar] [CrossRef] [Green Version]
- Thomas, H.; Humphreys, M.O. Progress and potential of interspecific hybrids of Lolium and Festuca. J. Agric. Sci. 1991, 117, 1–8. [Google Scholar] [CrossRef]
- Humphreys, M.W.; Thomas, H.M.; Harper, J.; Morgan, G.; James, A.; Ghamari-Zare, A.; Thomas, H. Dissecting drought-and cold-tolerance traits in the Lolium-Festuca complex by introgression mapping. New Phytol. 1997, 137, 55–60. [Google Scholar] [CrossRef]
- Humphreys, J.; Harper, J.A.; Armstead, I.P.; Humphreys, M.W. Introgression-mapping of genes for drought resistance transferred from Festuca arundinacea var. glaucescens into Lolium multiflorum. Theor. Appl. Genet. 2005, 110, 579–587. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Perlikowski, D.; Kosmala, A.; Rapacz, M.; Kościelniak, J.; Pawłowicz, I.; Zwierzykowski, Z. Influence of short-term drought conditions and subsequent re-watering on the physiology and proteome of Lolium multiflorum/Festuca arundinacea introgression forms with contrasting levels of tolerance to long-term drought. Plant Biol. 2014, 16, 385–394. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Tran, L.S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signaling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chan, Z. ROS Regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofo, A.; Dichio, B.; Xiloyannis, C.; Masia, A. Effects of different irradiance levels on some antioxidant enzymes and on malondialdehyde content during rewatering in olive tree. Plant Sci. 2004, 166, 293–302. [Google Scholar] [CrossRef]
- Campo, S.; Baldrich, P.; Messeguer, J.; Lalanne, E.; Coca, M.; San Segundo, B. Overexpression of a calcium-dependent protein kinase confers salt and drought tolerance in rice by preventing membrane lipid peroxidation. Plant Physiol. 2014, 165, 688–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharibi, S.; Tabatabaei, B.E.; Saeidi, S.G.; Goli, S.A.H. Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signalling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Dubey, A.K.; Kumar, N.; Kumar, A.; Ansari, M.A.; Ranjan, R.; Gautam, A.; Meenakshi Sahu, N.; Pandey, V.; Behera, S.K.; Mallick, S.; et al. Over-expression of CarMT gene modulates the physiological performance and antioxidant defense system to provide tolerance against drought stress in Arabidopsis thaliana L. Ecotoxicol. Environ. Saf. 2019, 171, 54–65. [Google Scholar] [CrossRef]
- Iqbal, N.; Hussain, S.; Raza, M.A.; Yang, C.Q.; Safdar, M.E.; Brestic, M.; Aziz, A.; Hayyat, M.S.; Asghar, M.A.; Wang, X.C.; et al. Drought tolerance of soybean (Glycine max L. Merr.) by improved photosynthetic characteristics and an efficient antioxidant enzyme activities under a split-root system. Front. Physiol. 2019, 10, 786. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Mittler, R. The roles of reactive oxygen species in plant cells. Plant Physiol. 2006, 141, 311. [Google Scholar] [CrossRef] [Green Version]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Shi, S.; Liu, Z.; Yang, F.; Yin, G. Drought tolerance in alfalfa (Medicago sativa L.) varieties is associated with enhanced antioxidative protection and declined lipid peroxidation. J. Plant Physiol. 2019, 232, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.M.; Pastori, G.M.; Driscoll, S.; Groten, K.; Bernard, S.; Foyer, C.H. Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J. Exp. Bot. 2005, 56, 417–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, H.M.; Harper, J.A.; Meredith, M.R.; Morgan, W.G.; King, I.P. Physical mapping of ribosomal DNA sites in Festuca arundinacea and related species by in situ hybridization. Genome 1997, 40, 406–410. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Hu, F.B.; Jia, Y.; Sha, H.; Zhao, H. Differential activity of the antioxidant defense system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci. Rep. 2019, 9, 8543. [Google Scholar] [CrossRef]
- Jin, R.; Wang, Y.; Liu, R.; Gou, J.; Chan, Z. Physiological and metabolic changes of purslane (Portulaca oleracea L.) in response to drought, heat, and combined stresses. Front. Plant Sci. 2016, 6, 1123. [Google Scholar] [CrossRef] [Green Version]
- Rao, E.; Chaitanya, V. Changes in the antioxidant intensities of seven different soybean (Glycine max (L.) Merr.) cultivars during drought. J. Food Biochem. 2020, 44, e13118. [Google Scholar]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Effects of yeast (Saccharomyces cerevisiae) supplementation on intake, digestibility, rumen fermentation and milk yield in Nili-Ravi buffaloes. Iran J. Vet. Res. 2018, 19, 96–100. [Google Scholar]
- Bil, A.; Fan, J.; Hu, Z.; Wang, G.; Amombo, E.; Fu, J.; Hu, T. Differential acclimation of enzymatic antioxidant metabolism and photosystem ii photochemistry in tall fescue under drought and heat and the combined stresses. Front. Plant Sci. 2016, 7, 453. [Google Scholar]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Jagła, M.; Rapacz, M.; Barcik, W.; Janowiak, F. Differential regulation of barley (Hordeum distichon) HVA1 and SRG6 transcript accumulation during the induction of soil and leaf water deficit. Acta Physiol. Plant. 2012, 34, 2069–2078. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Avramova, V.; AbdElgawad, H.; Vasileva, I.; Petrova, A.S.; Holek, A.; Mariën, J.; Asard, H.; Beemster, G.T.S. High antioxidant activity facilitates maintenance of cell division in leaves of drought tolerant maize hybrids. Front. Plant Sci. 2017, 8, 84. [Google Scholar] [CrossRef] [Green Version]
- Sheoran, S.; Thakur, V.; Narwal, S.; Turan, R.; Mamrutha, H.M.; Singh, V.; Tiwari, V.; Sharma, I. Differential activity and expression profile of antioxidant enzymes and physiological changes in wheat (Triticum aestivum L.) under drought. Appl. Biochem. Biotechnol. 2015, 177, 1282–1298. [Google Scholar] [CrossRef]
- Harb, A.; Awad, D.; Samarah, N. Gene expression and activity of antioxidant enzymes in barley (Hordeum vulgare L.) under controlled severe drought. Plant-Environ. Interact. 2015, 10, 109–116. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [Green Version]
- Lima, C.S.; Ferreira-Silva, S.L.; Carvalho, F.E.L.; Neto, M.C.L.; Aragão, R.M.; Silva, E.N.; Sousa, R.M.J.; Silveira, J.A.G. Antioxidant protection and PSII regulation mitigate photo-oxidative stress induced by drought followed by high light in cashew plants. Environ. Exp. Bot. 2018, 149, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Hossain, M.A.; Teixeria da Silva, J.A.; Fujita, M. Plant responses and tolerance to abiotic oxidative stress: Antioxidant defence is a key factor. In Crop Stress and Its Management: Perspectives and Strategies; Bandi, V., Shanker, A.K., Shanker, C., Mandapaka, M., Eds.; Springer: Berlin, Germany, 2012; pp. 261–316. [Google Scholar]
- Huseynova, I.M. Photosynthetic characteristics and enzymatic antioxidant capacity of leaves from wheat cultivars exposed to drought. Biochim. Biophys. Acta 2012, 1817, 1516–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Exogenous nitric oxide donor and arginine provide protection against short-term drought stress in wheat seedlings. Physiol. Mol. Biol. Plants 2018, 24, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Alché, J.D. A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol. 2019, 7, 101136. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; He, Z.; Chen, N.; Tang, Z.; Wang, Q.; Cai, Y. The roles of environmental factors in regulation of oxidative stress in plant. Biomed. Res. Int. 2019, 2019, 9732325. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [Green Version]
- Peltzer, D.; Dreyer, E.; Polle, A. Temperature dependencies of antioxidative enzymes in two contrasting species. Plant Physiol. Biochem. 2002, 40, 141–150. [Google Scholar] [CrossRef]
- Li, X.; Wei, W.; Li, F.; Zhang, L.; Deng, X.; Liu, Y.; Yang, S. The plastidial glyceraldehyde-3-phosphate dehydrogenase is critical for abiotic stress response in wheat. Int. J. Mol. Sci. 2019, 20, 1104. [Google Scholar] [CrossRef] [Green Version]
- Daszkowska-Golec, A.; Collin, A.; Sitko, K.; Janiak, A.; Kalaji, H.M.; Szarejko, I. Genetic and physiological dissection of photosynthesis in barley exposed to drought stress. Int. J. Mol. Sci. 2019, 20, 6341. [Google Scholar] [CrossRef] [Green Version]
- Majka, J.; Książczyk, T.; Kiełbowicz-Matuk, A.; Kopecký, D.; Kosmala, A. Exploiting repetitive sequences and BAC clones in Festuca pratensis karyotyping. PLoS ONE 2017, 12, e0179043. [Google Scholar] [CrossRef] [Green Version]
- Hurkman, W.J.; Tanaka, C.K. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986, 81, 802–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masojć, P.; Kosmala, A.; Perlikowski, D. Proteomic analysis of developing rye grain with contrasting resistance to preharvest sprouting. J. Appl. Genet. 2013, 54, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawłowicz, I.; Kosmala, A.; Rapacz, M. Expression pattern of the psbO gene and its involvement in acclimation of the photosynthetic apparatus during abiotic stresses in Festuca arundinacea and F. pratensis. Acta Physiol. Plant 2012, 34, 1915–1924. [Google Scholar] [CrossRef] [Green Version]
- Sibley, J.A.; Lehninger, A.L. Determination of aldolase in animal tissues. J. Biol. Chem. 1949, 177, 859–872. [Google Scholar] [PubMed]
- Willard, J.M.; Gibbs, M. Role of aldolase in photosynthesis. II demonstration of aldolase types in photosynthetic organisms. Plant Physiol. 1968, 43, 793–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 180–198. [Google Scholar]
- Yagi, K. Assay for serum lipid peroxide level its clinical significance. In Lipid Peroxides in Biology and Medicine; Yagi, K., Ed.; Academic Press: Cambridge, MA, USA, 1982; pp. 223–241. [Google Scholar]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Kosmala, A. Are nitric oxide donors a valuable tool to study the functional role of nitric oxide in plant metabolism? Plant Biol. 2011, 13, 747–756. [Google Scholar] [CrossRef]
- Doke, N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol. Plant Pathol. 1983, 23, 345–357. [Google Scholar] [CrossRef]
- Arasimowicz, M.; Floryszak-Wieczorek, J.; Milczarek, G.; Jelonek, T. Nitric oxide, induced by wounding, mediates redox regulation in pelargonium leaves. Plant Biol. 2009, 11, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Becana, M.; Aparicio-Tejo, P.; Irigoyen, J.J.; Sanchez-Diaz, M. Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiol. 1986, 82, 1169–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Gzyl, J.; Chmielowska-Bąk, J. Homocysteine over-accumulation as the effect of potato leaves exposure to biotic stress. Plant Physiol. Biochem. 2013, 63, 177–184. [Google Scholar] [CrossRef] [PubMed]
| F. arundinacea | Fa-HDT | high drought tolerant |
| Fa-LDT | low drought tolerant | |
| F. glaucescens | Fg-HDT | high drought tolerant |
| Fg-LDT | low drought tolerant |
| Gene | Primer Forward/Reverse | TaqMan Probe | Amplicon Length (bp) |
|---|---|---|---|
| Actin | GTCGAGGGCAACATATGCAA CCAGTGCTGAGCGGGAAT | TTCTCCTTGATGTCACGGAC | 65 |
| Ubiquitin | GCAAGAAGAAGACGTACA GACCTTGTAGAACTGGAG | CTTCACCTTCTTGTCCTTGTGCTT | 86 |
| pFBA | GAGACGTTCTACTACATG GAGGAGCTTGAGAGTGTA | TGTTCCTGTCCTTGCACTCGG | 140 |
| pPGK | CCTTGGTTGAGGAAGATAA CAGCAATGACAACATCAG | CTGGCAACAACTCTCCTGGC | 102 |
| pGAPDH | GTTCCAACACCAAATGTC TAGCCAAGAATGCCTTTC | CCTCTGATGCCGCCTTGATG | 125 |
| GR | GGGGAGTACGACTACGACCT TCGTAAGTCCACCCAAAGCC | GGCGGCGTCAGGGCCTCGCGCTT | 254 |
| GPX | TCACTCGGCGGCCTGGAGAA TTCACAGTGCGGGCTTACGA | CTACGCCACCGCCGCCACGGAGAA | 212 |
| APX | CTCGTATCGCAGGAGCTCG TTGGGCCACTCGCTAATGTT | CGGCTGCGGCTGGAGATGCGACGGC | 188 |
| Fe-SOD | TCTATCTCGGCGGTTCTCCA CCGTTGTTGTAGGCCTCCTT | GCTCGACACCAGCCCCTTCTACGGCCA | 219 |
| Cu/Zn-SOD | CCAGAGCATCCTCTTCGCC ATTGATGGAGGTGGAAGCCG | TCGCTCCGCCTCGTCTCCGCCCCC | 287 |
| Mn-SOD | TTGACGCCGCTGTCTCTAAGG TTTATCCAACGCCAGCCACA | GCTTCCGCCGTCGTCCAACTCCAGGGC | 266 |
| CAT | GTTCACCTTCCTCTTCGACG AAGTCGAACCTGTCCTCGTG | ACTACCGCCACATGGATGGCTCCG | 297 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lechowicz, K.; Pawłowicz, I.; Perlikowski, D.; Arasimowicz-Jelonek, M.; Majka, J.; Augustyniak, A.; Rapacz, M.; Kosmala, A. Two Festuca Species—F. arundinacea and F. glaucescens—Differ in the Molecular Response to Drought, While Their Physiological Response Is Similar. Int. J. Mol. Sci. 2020, 21, 3174. https://doi.org/10.3390/ijms21093174
Lechowicz K, Pawłowicz I, Perlikowski D, Arasimowicz-Jelonek M, Majka J, Augustyniak A, Rapacz M, Kosmala A. Two Festuca Species—F. arundinacea and F. glaucescens—Differ in the Molecular Response to Drought, While Their Physiological Response Is Similar. International Journal of Molecular Sciences. 2020; 21(9):3174. https://doi.org/10.3390/ijms21093174
Chicago/Turabian StyleLechowicz, Katarzyna, Izabela Pawłowicz, Dawid Perlikowski, Magdalena Arasimowicz-Jelonek, Joanna Majka, Adam Augustyniak, Marcin Rapacz, and Arkadiusz Kosmala. 2020. "Two Festuca Species—F. arundinacea and F. glaucescens—Differ in the Molecular Response to Drought, While Their Physiological Response Is Similar" International Journal of Molecular Sciences 21, no. 9: 3174. https://doi.org/10.3390/ijms21093174





